| Equilibrium concentration of extraction as \(t \rightarrow \infty \) | |
|---|---|
| Rate constant of extraction process | |
| Time |

Photo credit to Kimzy Nanney
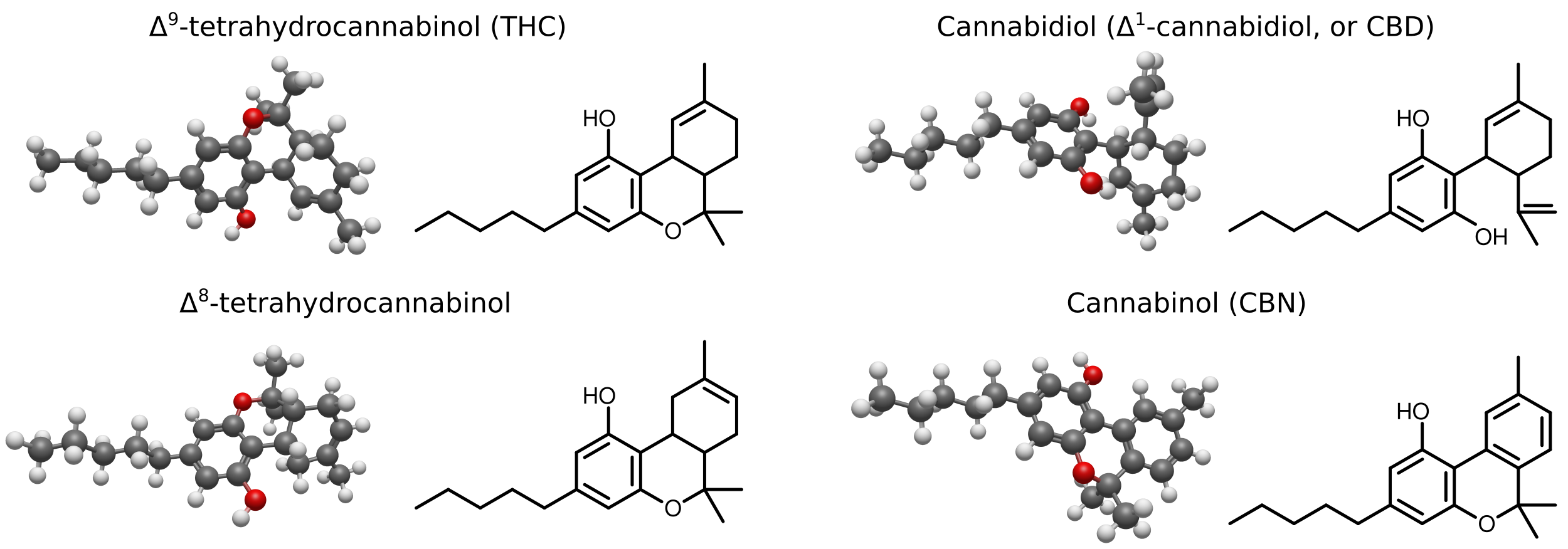
[Caption] Structure of the four most studied and common cannabinoids. Δ9-THC is the main psychoactive molecule responsible for cannabis intoxication while Δ8-THC is the lesser known, less potent, less concentrated variant. Cannabidiol (or CBD oil) has central nervous system effects (well documented treatment of certain kinds of epilepsy, anxiety relief, etc) but is not responsible for intoxication. CBD is seldom referred to in scientific literature using Δ notation (as in Δ1-CBD) because other CBD variants (like Δ6-CBD, see the explanation on the confusing numbering pattern below) are discussed so rarely and are naturally present in such small amounts. Cannabinol is weakly psychoactive, present in cannabis in only trace amounts. It is a decomposition product of THC and CBD in the presence of heat and/or light. Black, red, and white indicates carbon, oxygen, and hydrogen atoms, respectively. These cannabinoid molecules are structural isomers with 23 non-hydrogen atoms.
Cannabis is the subject of much confusion partly because of its illegal status and partly because of its vociferous supporters who will repeat and mischaracterize any positive scientific data while ignoring any negative data. This article will discuss four questions which are the subject of much misinformation: What is cannabis/marijuana/sativa/indica? How dangerous is it? Why does cannabis cause dry mouth? What exactly does a drug test detect? And what is decarboxylation and why do all the recipes for edibles mention it with the clarity of superstition?
Despite what some states are doing to legalize cannabis, as of the time of this writing, cannabis is illegal in the US at the federal level so it is a federal crime to possess, transport, or sell everywhere in the US. The merits and drawbacks of its legal status are highly contentious and outside the scope of this article. Like all posts on this blog, the article focuses on the quantifiable or at least objective aspects of the subject.
This article cites little original research. Approximately 4,000 peer-reviewed papers on cannabis are published every year. To write a proper literature review on subject matter of this breadth requires a far higher word count and more subject matter expertise than I have. Therefore, this article cites mostly review articles, peer-reviewed articles which present a subject matter expert's view of the state of the science. The length of this article in relation to the other articles on drugs reflects the amount of circulating misinformation and in no way reflects an enthusiasm or recommendation for usage.
Cannabis has been used by humans since at least 3000 BC [1,2,3]. It is tied with caffeine for the title "second oldest drug in human history" (the oldest drug being alcohol). It has been banned by various governments with varying levels of success since 1300 AD [4]. The dominant active compound of cannabis, tetrahydrocannabinol (THC), was discovered in 1964 [5]. It was synthesized in 1965 [6]. While naturally occuring THC has been illegal in the US at the federal level since 1970, synthetic THC was first approved by the FDA as an AIDs medication in 1992 [7].
Cannabis is a plant and a collection of molecules (cannabinoids) many of which have psychoactive effects. Of the most commonly used recreational drugs (alcohol, tobacco, caffeine, and cannabis), cannabis is the most unique. It is the only one composed of a heterogenous mix of psychoactive compounds, the only one whose primary active compound (THC) has multiple chiral centers, the only one illegal at all ages in the US, and the topic of the most misinformation (on both the pro-cannabis and anti-cannabis sides of the debate). Discussions of THC or cannabis often take place between two parties who are unclear about the basic complexities of the topic. Is cannabis a family or species? What are cannabinoids? Which isomer of THC are we talking about?
Biologists categorize life by kingdoms, clades, orders, families, genera, and species. Cannabis is a genus from the family Cannabaceae, order Rosales, clade Angiosperms, and kingdom Plantae. Within the genus are 3 species (though the categorization of these species is the subject of some academic debate [8]): Cannabis sativa, Cannabis indica, and Cannabis ruderalis [9]. Sativa and indica generally have more THC and CBD [10,11,12] and are therefore more effective recreational drugs; ruderalis has been largely ignored in the recreational market except in crossbreds where it contributes desirable flowering properties. Within each species are an ever-changing number of strains which vary in their composition of psychoactive compounds. The term marijuana is a remnant of yellow journalism and political calculation from the 1930s attempting to associate drug use with "Negroes, Hispanics, Filipinos, and entertainers," "satanic music, jazz, and swing," and interracial sexual relations. We have Henry Anslinger, the father of the modern war on drugs, to thank for all those quotes [13]. To use the term marijuana is to abandon the language of science in favor of the language of prohibitionists.
[Caption] Two carbon numbering schemes of cannabinoids illustrated with THC [14] and CBD [15]. The dibenzopyran system is the most common one, especially recently. It is the number scheme used in this article unless otherwise stated. A pyran is a 6 membered ring with one oxygen, 5 carbons, and two double bonds. Ring B is a pyran. Rings A and C are benzenes (six carbon ring with 3 double bonds, or six sp2 hybridized carbons for the o-chem nerds) in cannabinol. Hence the description of THC as a dibenzopyran. CBD follows the monterpenoid numbering scheme even in modern papers [15,16]. Obviously not all cannabinoids have double bonds in all the right places (THC is missing two on ring C) but no nomenclature is perfect. Older papers (1960s-1970s) such as those on the discovery [5] and synthesis [6] of THC use the monoterpenoid scheme. A monoterpene is a terpene composed from 2 isoprenes creating a 10 carbon structure. To most easily see the terpene structure of cannabinoids, compare the structure of CBD to limonene. The terpene is composed of ring C (carbons 1-6) and carbons 7, 8, 9, and 10 in the monoterpenoid numbering system. Also know that some papers use 9s and 0s in place of ' and '' respectively. In these papers 1' = 19, 2' = 29, etc, and 1'' = 10, 2'' = 20, etc. This is uncommon but when a paper references carbon 29, it's referring to 2' [16].
The high of cannabis is caused by interactions with endocannabinoid receptors in the body. Unsurprisingly, those receptors are in the body because they interact with molecules the body naturally produces. If the receptor stimulator was synthesized by the body, it is an endogenous cannabinoid. If the stimulator comes from a synthetic chemistry lab, it is a synthetic cannabinoid. If it comes from a plant, it is a phytocannabinoid. The primary (but not only) phytocannabinoid responsible for the high from cannabis use is THC.
Chemists have historically used two different numbering conventions to identify the carbon atoms of THC — the monoterpenoid system and dibenzopyran system. The dibenzopyran system is the most common, particularly in recent history so this article will use the dibenzopyran system unless otherwise stated. Older papers refer to Δ1-THC (monoterpenoid system) which is the same compound as Δ9-THC (dibenzopyran system). Cannabinoid chemistry began in 1964 using the monoterpenoid system to describe the discovery of THC [5]. The earliest mentions of the dibenzopyran system I can find is from 1965 [17] in a UN report which discussed five competing number conventions at the time. Three of the conventions swiftly feel out of favor (I never once ran across them until finding them in that report, except here [18]). The monoterpenoid system has been used in the title of paper as late as 2004 [19] but since at least 1985 or 1990, the dibenzopyran system is dominant numbering convention.
THC and CBD are numbered differently because modern papers use still use the monoterpenoid numbering system to describe the carbons of CBD but the new dibenzopyran system to describe THC. THC and CBD are structural isomers which vary by a single bond. The 23 major atoms of both molecules are in the same relative positions and yet the numbering schemes are completely different.
Stereochemistry, a subset of the study of isomers, is the study of the relative spatial arrangements of atoms within molecules. It is most common in discussion of organic (carbon based) chemistry. Because chemical structures are normally described and sketched in 2D but the phenomenona being discussed are a consequence of 3D relationships, stereochemistry challenges most people's spatial reasoning. It is important because even if two molecules are composed of the same atoms, and all those atoms are bonded to the other atoms in the same order, they may still have different structures because of differences in the geometric arrangements of those bonds. Sometimes this has significant biological consequences. For example, 19 of the 20 amino acids have a chiral center. They are described as L- or D- where D- is the configuration synthesized by all known lifeforms. The L- configurations of some amino acids are toxic; for others, the effects of administering L- forms are blunted compared to the D- forms [20]. Stereochemistry is often overlooked in the field of cannabinoids and this creates ambiguous terminology.
Isomers are chemicals with the same number and types of atoms but which are arranged differently. For example, butane and isobutane have the same chemical formula, C4H10, but they have different structures. These are "structural isomers" because on a simple 2D projection, we can trace out which bonds have changed. Δ9-THC, Δ8-THC, Δ6-CBD and CBD (Δ1-CBD) are structural isomers all having the formula C21H30O2.
[Caption] Structural isomers n-butane and isobutane.
The more complex type of isomers are stereoisomers which are chemicals that appear identical when drawn in 2D but are not actually superimposable. For example, your right and left hands are "stereoisomers". All of the same bones are connected in the same order but no matter how you orient your hands, you cannot superimpose one atop the other. Carbon atoms work the same way. Because carbon can bond to four unique groups, each carbon can have up to two orientations. Most carbon atoms have only one orientation because most of them are bonded to at least two identical things. For example, each carbon in n-butane above is bonded to either two or three hydrogens so there are no stereoisomers of n-butane. However if one of the carbons in the middle were bonded to -OH instead of H, that carbon would have four unique bonds (-H, -OH, -CH3, -CH2CH3) and it would have two stereoisomers. The molecule I just described is 2-butanol and its wikipedia page begins with a description of its stereochemistry.
[Caption] Illustration of the chiral centers of pairs of stereoisomers. [Left] Stereoisomer illustration where carbon (C) is bonded to A, B, D, and E (not elements, just placeholders for illustration). Notice that if you switch the positions of any two groups bonded to C, the objects become superimposable again. Pair-swapping any two groups creates a mirror image of the original object. [Right] Same illustration but depicting 2-butanol focusing on the chiral carbon.
Each carbon atom with four unique bonds is known as a chiral center. Because each chiral center's orientation is independent of the others, and each chiral center has two orientations, the number of stereoisomers is \(2^n\) where \(n\) is the number of chiral centers. If all of the chiral centers in one molecule are in the opposite configuration of another molecule, those molecules are referred to as enantiomers. If there is only one chiral center, the two stereoisomers are, by definition, enantiomers. Pairs of enantiomers are mirror images of each other.
Many introductions to stereochemistry illustrate its importance by the thalidomide example. Much of what is described in that example is untrue or exaggerated. However, stereochemistry undoubtedly has important effects on the nature of biological interactions. Most stereoisomers do not interconvert in the body. It is therefore important that, regardless of whether a drug interconverts or not, we understand what stereoisomers exist, use our language precisely to describe the molecules of interest, learn how they interact with the body, and determine whether or not they interconvert under physiological conditions.
THC has two chiral centers at carbons 6a and 10a (dibenzopyran numbering system) and therefore four stereoisomers. (-)-trans-Δ9-THC (often just THC) is the biologically synthesized isomer. The three other stereoisomers of Δ9-THC have been synthetically produced: (+)-trans-Δ9-THC, (-)-cis-Δ9-THC, and (+)-cis-Δ9-THC. Even in the scientific literature, it is rare that authors acknowledge the stereochemistry of the THC they are studying (sometimes THC means (-)-trans-Δ9-THC, the naturally occurring stereoisomer, sometimes it refers to a racemic mixture). When reading papers, the origins of the THC can help clarify the definition. If the THC is isolated from a plant, it is (-)-trans-Δ9-THC; if a chemist synthesized it and there is no mention of isomer purification, it is a racemic mixture.
[Caption] The four stereoisomers resulting from the 2 chiral centers of Δ9-THC. Bonds shown as wedges in the stereocenters are highlighted in red to emphasize the differences between the stereoisomers. Depending on the resource, the term THC may refer to only the left most compound or a mixture of all four. Dronabinol, the commercial synthetic THC product, is a racemic mixture of all four stereoisomers of THC.
Cannabis is the only recreational drug defined as a potpourri of a family of psychoactive chemicals. (Other recreational drugs are often combinations but this is due to dilution of cocaine or heroin with other white powders like caffeine, baking soda, or sugar [21,22,23]. The cocaine or heroin themselves are single identified molecules, not combinations.) The variety of strains of cannabis available produce a variety of psychoactive effects because the plant produces at least 100 known cannabinoids [24]. Some cannabinoids, like THC, are highly psychoactive and at high doses, mildly hallucinogenic. Others like cannabidiol (commonly known as CBD oil) have almost undetectable psychoactivity. Some combinations of cannabinoids cause more dry mouth than others, some induce more anxiety in certain people (although anxiety has highly multivariate causes including disposition and context), and some combinations cause different subjective sensations. This pharmacological variety justifies an enthusiasm for exploration unlike any other drug.
Despite this justifiable enthusiasm, cannabis is undeniably dangerous, particularly when smoked even though current evidence suggests it may be less toxic than tobacco [25]. The halflife of cannabinoids in the body is much longer than nicotine so cannabis users smoke less plant matter per day than tobacco smokers. This difference in inhaled volume of particulates and carcinogens is one possible reason cannabis smokers appear to be at less risk of pulmonary diseases than tobacco smokers. Because cannabis is difficult to study, partly because of its illegal status and partly because of confounding population factors (cannabis smokers are more often also tobacco smokers than the general population), reliable long-term studies linking cannabis smoking to health outcomes are not available. What studies are available are mostly survey studies which consistently link habitual cannabis smoking and chronic bronchitis [26]. Current science is inconclusive regarding cannabis and obstructive lung disease (the hallmark disease of tobacco smoke) and only some studies find a connection between cannabis and lung cancer [27]. However, every product of combustion which has been extensively studied has been proven to be both carcinogenic and toxic to the lungs. Furthermore, tobacco and cannabis are extremely similar plants with the exception of nicotine and cannabinoids, so most of the same carcinogens in tobacco smoke are in cannabis smoke as well. For this reason, any claim that cannabis smoke is free from negative consequence should be viewed with the same suspicion we apply to claims about snake oil, healing crystals, and other unproven health claims.
Low-risk cannabis-use guidelines (LRCUG) [28] consistently recommend only using non-smoked cannabis products to avoid carcinogenic or pulmonary toxic combustion products, avoiding synthetic cannabinoids, abstaining until at least after age 16, and keeping total intake low by only using low doses infrequently. This is not, however, a glowing endorsement of THC edibles. Deleterious effects from regular common doses of edible THC are not yet documented in the medical literature (that I was able to find in 2019). Separating the effects of edible THC from smoked cannabis is difficult because of the rareness of edible-only THC users. If there are chronic toxicity problems associated with THC ingestion, we have not yet been able to study them.
Cannabis advocates will often claim that cannabis cannot kill anyone from acute exposure. Strictly speaking, this is untrue though it is extremely difficult and requires synthetic cannabinoids, multi-drug toxicity, or severe underlying conditions. In the rare event of fatal acute cannabis poisoning, the documented mechanism is cardiovascular toxicity (as might be expected of a drug that induces tachycardia), either ischemic strokes or heart attacks. In a few cases where it was possible to measure, acute toxicity has been associated with arrhythmias, either Brugada's syndrome (cases 18 and 20 in Table 1 of [29]) or ST elevation (case 3 in Table 1 of [29]), which can cause heart failure in extreme cases. Also remember, whenever we discuss the acute toxicity of cannabis, it is important to remember we are discussing medical case reports, the style of report doctors use to document fantastically unlikely occurrences, things that may occur less than once in a doctor's career. Someone, somewhere in the last 50 years has died from an unlikely reaction to every normally-benign over-the-counter medication ever sold.
Δ9-THC was first synthesized in 1965 [6]. Synthetic THC is marketed under the trade name Dronabinol and has been approved by the FDA to treat anorexia in AIDs patients since 1992 [7]. Some resources claim that Dronabinol is a specific stereoisomer of THC but the World Health Organization lists all 4 stereoisomers as possible components of Dronabinol [30]. The current cat and mouse race between chemists looking to supply recreational drug users and drug enforcement officials has produced hundreds of synthetic cannabinoid receptor agonists with largely unknown safety profiles. The EU's Early Monitoring System for new psychoactive substances is currently monitoring over 130 synthetic cannabinoids [31], their fastest growing class of new psychoactive substances. Ironically, given that these compounds are often taking hold among communities which prize 'natural' products, most synthetics are dissolved in organic solvents like acetone and mixed with various herbs before the solvents are evaporated off leaving behind synthetic cannabinoids.
[Caption] Various common [31] synthetic endocannabinoid receptor agonists shown next to Δ9-THC for comparison. The 5 carbon chain which is common to most cannabinoids and appears to be partially responsible for receptor interactions is shown in blue.
Anyone who has ever been dehydrated understands the unpleasantness of dry mouth but dry mouth can be caused by all kinds of things not related to hydration. It is the most common negative side of cannabis regardless of the route of administration. There are two theories on how cannabinoids interfere with saliva production, the anticholinergic mechanism and the endocannabinoid receptor mechanism. Let's review what causes saliva production and how it is most commonly interrupted.
There are six major salivary glands around the mouth (two parotid glands, two submandibular glands, and two sublingual glands) and hundreds of smaller unnamed glands. There are subtle differences in the fluid composition and degree to which each gland responds to nerve stimulation but for this article we will neglect those differences. In the absence of nerve stimulation from the parasympathetic system (the "rest and digest" system), saliva is produced at too low a rate to avoid dry mouth.
To maintain oral lubrication, nerve signals from the parasympathetic nervous system are relayed to the gland from the nerve terminal via a neurotransmitter molecule called acetylcholine. Once acetylcholine leaves the nerve terminal it begins interacting with the gland receptors and acetylcholinesterase begins to degrade it. Any drug that prevents the release of acetylcholine from the nerve terminal, interfers with acytelcholine's receptor interactions, or overly stimulates the degradation of acetylcholine is an anticholinergic drug. Anticholinergic effects are a common side effect of many pharmaceuticals (Benadryl and many antidepressants come to mind). Some drugs' intended effect is anticholinergic — tropicamide, a drug used by optometrists and opthalmalogists to dilate pupils, blocks the action of a specific acetylcholine receptor.
Cannabinoids are occassionally reported to have anticholinergic effects and sometimes even anticholinergic toxicity in medical literature [32] and dry mouth is a widely recognized complication of cannabis use regardless of the route of administration. UpToDate, the smartphone app your doctor relies on for the most current research and best practices in a clinical setting, lists nine symptoms of acute cannabis intoxication [33]: tachycardia, increased blood pressure (or especially in the elderly - orthostatic hypotension), increased respiratory rate, conjunctival injection (red eye), dry mouth, increased appetite, nystagmus, ataxia, and slurred speech. Of those symptoms, four are symptoms of anticholinergic poisoning. Grotenhermen [34] describes the dry mouth of cannabis as an "atropine-like effect" (atropine being the prototypical anticholinergic drug used to introduce the topic of anticholinergics to medical students).
There is a dearth of peer reviewed information in the medical literature on the mechanisms and effects of cannabis, particularly the psychoactive cannabinoids which are highly illegal. Because of the red tape preventing funding to studies of the mechanisms, effects, and variability of cannabis, absence of evidence is less evidence of absence than it might normally be.
There are pop science articles online claiming that saliva production is inhibited via endocannabinoid receptor interactions within the salivary glands (having nothing to do with parasympathetic nerves or acetylcholine). The science in these articles ranges from dubious to factually incorrect. The endocannabinoid receptor mechanism of dry mouth is not supported in any peer-reviewed literature I have been able to locate beyond one obscure Argentinian study on rat glands [35] which is often cited by those who think the endocannabinoid receptor system is the key to life, the universe, and everything. While it is certainly possible that the presence of endocannabinoid receptors in the salivary glands play a key role in dry mouth, my reading of the medical literature in 2019 did not find any conclusive support for that theory. But again, absence of evidence is less evidence of absence than it might normally be when dealing with highly-regulated, difficult-to-study drug mechanisms.
Cannabinoids are synthesized in cannabis plants from hexanoyl-CoA via a series of enzymatically catalyzed reactions. Hexanoyl-CoA is a medium-chain fatty-acid found in all living things from bacteria to human cells to cannabis [36].
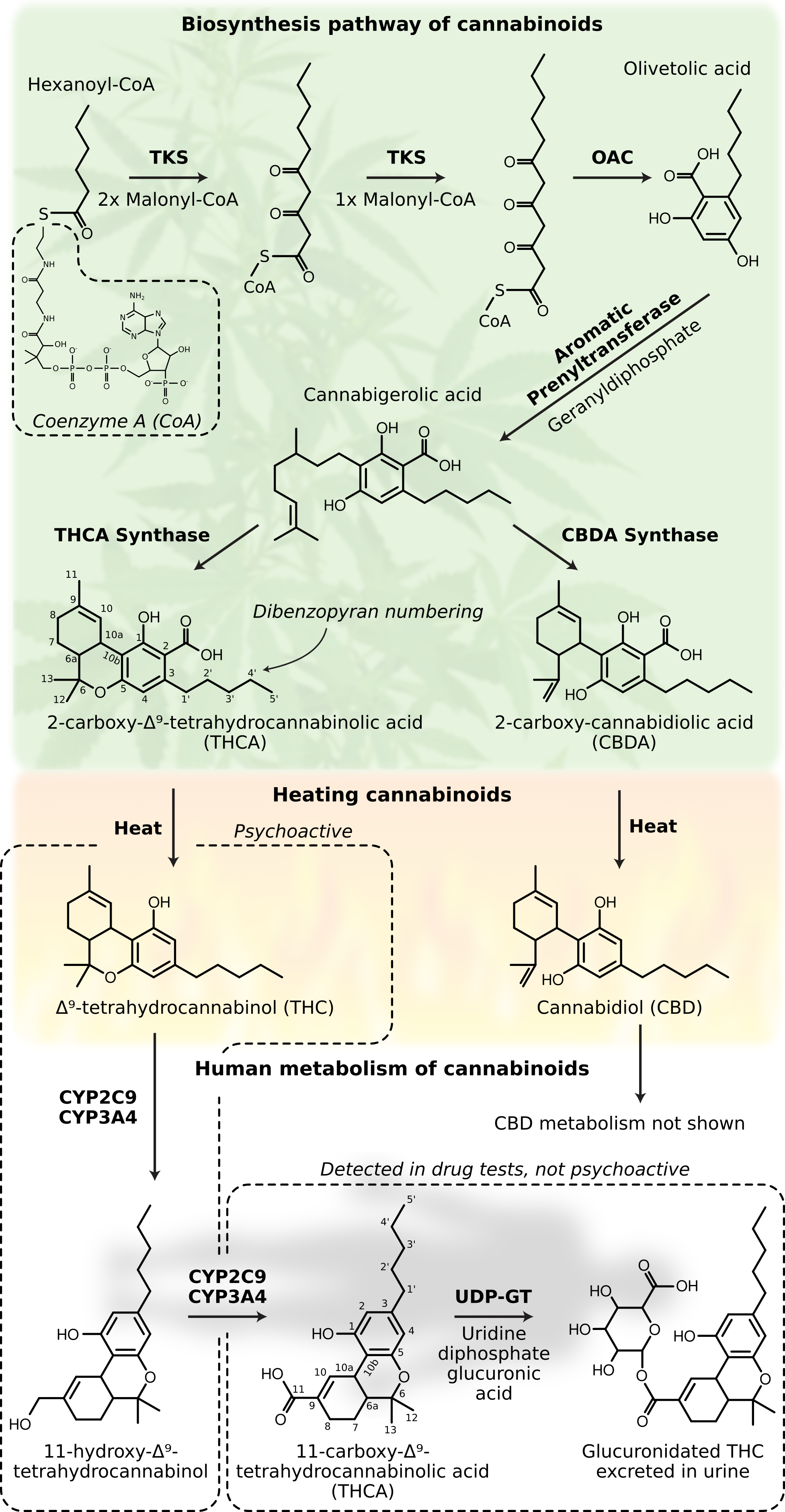
[Caption] Side reactions not shown. Reaction pathways, especially in complicated systems like those found in biology, are filled with side reactions. Some are known and omitted for space reasons; many more are unknown. These side reactions are responsible for the variety of naturally-occuring cannabinoid compounds. Biosynthesis pathway of cannabinoids from [37], structure of coenzyme A from [38]. Biosynthesis of cannabinoids begins with hexanoyl-CoA, a fatty-acid common to all living cells [36]. A series of enzyme catalyzed reactions converts hexanoyl-CoA to cannabigerolic acid. Here the pathway splits, THCA synthase produces tetrahydrocannibolic acid (THCA) and CBDA synthase produces cannabidiolic acid (CBDA). Enzyme catalyzed reactions are unusual in their ability to produce nearly pure stereoisomers. The THCA produced in cannabis plants is overwhelmingly (-)-trans-Δ9-THCA (see earlier discussion of the stereochemistry of THC). Heating cannabis converts the THCA and CBDA to THC and CBD, respectively. The acid precursors are not psychoactive so this step is critical in the synthesis of psychoactive compounds. The body metabolizes THC via the CYP450 enzyme system, specifically CYP2C9 and CYP3A4 [39]. Glucuronidation structure from [40]. Enzyme names or abbreviations are indicated in bold, reactants in plain text. Abbreviations: tetraketide synthase (TKS), olivetolic acid cyclase (OAC), tetrahydrocannibolic acid synthase (THCA synthase), cannabidiolic acid synthase (CBDA synthase), uridine 5'-diphospho-glucuronosyltransferase (UDP-GT), coenzyme A (CoA). The abbreviation THCA is used to describe two different molecules, 11-carboxy-THC and 2-carboxy-THC, depending on the context. In plant biology, or discussions about decarboxylating cannabis for edibles, THCA refers to 2-carboxy-THC. In the context of drug testing, THCA refers to 11-carboxy-THC. Clipart sources, cannabis.
There is a lot happening in the above figure. To summarize, Cannabis plants start with a common fatty-acid and synthesize THC-acid and CBD-acid via a series of enzyme-catalyzed reactions. Heat from burning or baking the cannabis removes the acid leaving THC and CBD. The acid precursors and CBD are not psychoactive but the THC is. THC is then converted by the liver (via CYP enzymes) to 11-hydroxy-THC and then to 11-carboxy-THC. The 11-carboxy-THC is glucuronidated and then excreted in the urine. The graphic above does not show the human metabolic pathway for removing CBD but it is similar. CYP2C9 and CYP3A4 metabolize THC, CBD, and CBN. CYP2C9 plays a smaller role in CBD metabolism compared to THC [41]. CBN and its acid precursors (not pictured) are not directly synthesized by cannabis plants; they are a breakdown product of other cannabinoids in the presence of light and heat.
The psychoactive compounds here are THC and 11-hydroxy-THC [42,43]. 11-hydroxy-THC is qualitatively three times more potent than THC [44], not the sometimes claimed ten times. Because of first-pass metabolism, edible cannabis increases the ratio of 11-hydroxy-THC:THC in the blood. The difference in this ratio is likely responsible for the qualitatively different highs of smoked vs oral cannabis.
Workplace drug testing for cannabis use has several possible targets: THC, 11-hydroxy-THC, 11-carboxy-THC, and glucuronidated THC. Drug testing for cannabis began in 1986. Then as now, the tests directly detect 11-carboxy-THC in the urine [45]. However, prior to running the test, glucorinated-THC is converted back to 11-carboxy-THC via enzymes or alkaline hydrolysis. Because the extent of conversion of glucorinated-THC to 11-carboxy-THC is poorly controlled and because the half-lives of cannabinoids in the body are highly variable, testing for cannabinoids in urine is inconsistent [46]. As of 2010, according to the US federal government, the threshold concentration of 11-carboxy-THC for a positive test is 15 or 50 ng/mL for a confirmation or screening test, respectively [47]. These tests do a reasonable job of detecting cannabis use within recent days or weeks. However, when the question is a matter of impairment, the tests fall short. 11-carboxy-THC is not a psychoactive compound but it is the only compound directly detected on a standard urine test. Directly detecting THC does not solve this problem either. In regular cannabis users, THC is readily detected in blood plasma by GC-mass spectrometry even after a full week of abstinence [48], long after the impairing effects have passed.
CBD and its byproducts should not cause positive results on cannabis tests. However, as of this writing, a significant portion of "pure" CBD products are contaminated with THC [49,50] or synthetic cannabinoids [51]. Misconceptions about CBD are common but CBD alone does not cause psychological symptoms associated with the high from cannabis [52]. If your CBD product gives you a high, it has more than CBD in it. However, there are CBD derivatives with psychoactivity [53] including Δ6-CBD (the analog of Δ8-THC) [54].
On the brightside, experiments to develop and validate testing procedures have resulted in some truly amazing experiments. The following is directly copied from the methods section of a published peer-reviewed paper: "Six adult male daily cannabis smokers resided on a closed clinical research unit. Oral THC capsules (20 mg) were administered every 4-8 h in escalating total daily doses (40-120 mg) for 7 days." These scientists [55] literally locked six stoners in a hotel room and fed them edibles every 4 hours for a week straight! If you ever needed a paper to motivate your rebellious teenager in school, here it is.
The majority of psychoactive compounds in cannabis exist in an inactive state which cannot interact with endocannabinoid receptors and therefore have no psychoactive effects. To make psychoactive compounds from cannabinoid acids requires the removal of a carboxyl group from the C2 carbon via the application of heat (a decarboxylation reaction). Further heating also causes the degradation and evaporation of THC so there is a window in time and temperature which maximizes the yield of psychoactive cannabinoid compounds [56].
Cannabis smokers do not spend much time thinking about decarboxylation. It happens in an uncontrollable way as a consequence of burning cannabis so there is no debate about which method produces the highest yield of psychoactive compounds. Approximately 30% of the THCA in the biomass is converted to THC via smoking. With precision lab equipment, the yield may reach 70% [57]. The preparation of edibles is involves more skill than smoking. To create a THC-filled baked-good from cannabis flowers or leaves, the baker begins with cannabinoid acids in the plant matter and ends with solutions of cannabinoids in oil in pastries. This requires an extraction step (moving the molecules from plant matter to an oil solution) and a decarboxylation step (removing the carboxylic acid group).
[Caption] Decarboxylation pathway from [56,57]. CBD can be converted to THC via ethanol+HCl or BF3 [54]. Methods for converting CBD to THC involving acids or catalysts have been patented since 2008 [58]. Modern patents avoid highly acidic reagents and claim higher conversions [59].
The next decision the baker makes is whether to do the extraction and decarboxylation in one step or two. If the procedure is done in two steps, the higher yield order is decarboxylation first because cannabinoid oils are more soluble in oil than cannabinoid acids.
Extraction procedures have several variables to consider: (1) type of oil, (2) temperature, (3) time, and (4) the surface area to volume ratio of the solid phase. (1) I have not found any evidence to suggest the superiority of any specific cooking oil. The oil is usually selected based on lore circulating within a baker's social circle or for its other desirable properties in the cooking process. (2) Higher temperatures increase the diffusivity of all molecules and increase the solubility in liquids relative to solids. Sufficiently high temperatures can reduce the yield through evaporation and degradation of THC. (3) Extractions follow exponential curves, \( C_\text{final}\left( 1-e^{-kt} \right)\), where \(k\) depends on all of the factors above. Depending on conditions, it may or may not be materially useful to double the extraction time in the hopes of creating a more potent product. However, if doubling the extraction time does not make significant improvements to a recipe, quadrupling the extraction time will have no discernible effect. (4) In all extractions, increasing the surface to volume ratio increases total flux of molecules out of the solid phase. For this reason, ground cannabis facillitates the extraction of cannabinoids into the oil phase faster than whole leaves or flowers.
Decarboxylation depends on temperature and time. Like all hydrocarbons, however, they burn in the presence of enough oxygen and heat. More practically, cannabinoids degrade with heat and time. Specifically, THC degrades to cannabidiol in the presence of heat, oxygen, or/and light [57]. THC degradation can be prevented or slowed to insignificant rates by heating THCA in a dark vacuum oven (no light or oxygen) [56]. Also reducing yields, cannabinoids evaporate beyond 155-160°C (310-320°F). The decarboxylation reaction of THCA follows first order kinetics. A review paper of decarboxylation kinetics mapped the time-temperature space to "maximal conversion" from a variety of papers and experimental conditions. The true kinetics of the process depend on the composition of the atmosphere, the surface exposed to the atmosphere, and any experimental shortcomings. For this reason, the review presented the time to maximal conversion as a range of times.
[Caption] Graph of decarboxylation conversion vs time reproduced from [60].
Ovens are a common but poorly controlled tool for decarboxylation because an oven set to 350°F does not hold its contents at 350°F. The air is a slow mechanism of heat transfer and the walls radiate heat in uneven ways. The water content in the plant means the plant will locally experience the wet-bulb temperature of the oven but the controls on the oven govern only the dry-bulb temperature. Ovens are therefore subject to variability with changing humidity and elevation. All these flaws make uniform reliable decarboxylation nearly impossible. Fortunately, the target window of time and temperature is sufficiently wide that even despite these flaws, it is possible to produce potent decarboxylated THC in a home oven.
The most reliable preparations of THC oils outside a scientific lab environment are those produced by sous vide devices or slow cookers. These methods combine extraction and decarboxylation into one step with tight controls on temperature and time. Since the only variability is the composition of the cannabis, these methods are the most reproducible.
According to the wonderfully named website priceofweed.com, cannabis costs approximately $100-$300 per oz depending on where you buy it in the US. One oz of cannabis will keep a functional stoner happy for between one and four weeks. Like all vices, the expense of cannabis varies depending on the extent of use. An all consuming habit can take $10,000 or more per year to finance though most users consume less than $1,000 per year.
[1] "Cannabis in Eurasia: origin of human use and Bronze Age trans-continental connections," Vegetation History and Archaeobotany, vol. 26, pp. 245—258, 2017.
[2] "The origins of cannabis smoking: Chemical residue evidence from the first millennium BCE in the Pamirs," Science Advances, vol. 5, pp. eaaw1391, 2019.
[3] "Cannabis and Frankincense at the Judahite Shrine of Arad," Journal of the Institute of Archaeology of Tel Aviv University, vol. 47, pp. 5-28, 2020.
[4] "Neurobehavioral toxicology of substances of abuse," in Addiction Medicine: Science and Practice, pp. 303, 2011.
[5] "Isolation, structure, and partial synthesis of an active constituent of hashish," Journal of the American Chemical Society, vol. 86, pp. 1646—1647, 1964.
[6] "A total synthesis of Delta-1-tetrahydrocannabinol, the active constituent of hashish," Journal of the American Chemical Society, vol. 87, pp. 3273—3275, 1965.
[7] "Development of Cannabinoid Drugs," in Marijuana and Medicine: Assessing the Science Base, 1999.
[8] "Cannabis confusions," Bmj, vol. 332, pp. 175—176, 2006.
[9] "A numerical taxonomic analysis of Cannabis with special reference to species delimitation," Systematic Botany, pp. 67—84, 1976.
[10] "Chemotaxonomy of Cannabis I. Crossbreeding between Cannabis sativa and C. ruderalis, with analysis of cannabinoid content," Economic Botany, vol. 32, pp. 387, 1978.
[11] "A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae)," American Journal of Botany, vol. 91, pp. 966—975, 2004.
[12] "An overview of products and bias in research," Neurotherapeutics, vol. 12, pp. 731—734, 2015.
[13] "Book Review: Marijuana Law, Policy, and Authority By Robert A. Mikos, Professor of Law, Vanderbilt University Law School," 2017.
[14] "Taxonomy of Cannabinoids," in Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential, pp. 16, 2002.
[15] "Chemistry and Structure Activity Relationships for Tetrahydrocannabinols and Endocannabinoids," in Cannabinoids, pp. 132, 2003.
[16] Figure 1 from, "Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy," Cannabis and Cannabinoid Research, vol. 1, pp. 90—101, 2016.
[17] "E/CN. 7/479: The question of cannabis; cannabis bibliography," United Nations. Commission On Narcotic Drugs, 1965.
[18] "Recent Developments in Cannabis Chemistry," Journal of Psychedelic Drugs, vol. 2, pp. 15-29, 1968.
[19] "The gene controlling marijuana psychoactivity molecular cloning and heterologous expression of Δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L.," Journal of Biological Chemistry, vol. 279, pp. 39767—39774, 2004.
[20] "D-amino acids in nature, agriculture and biomedicine," All Life, vol. 13, pp. 11—22, 2020.
[21] "The hidden web and the fentanyl problem: Detection of ocfentanil as an adulterant in heroin," International Journal of Drug Policy, vol. 40, pp. 78—83, 2017.
[22] "Analytical studies on illicit heroin," Pharmaceutisch Weekblad, vol. 9, pp. 203—211, 1987.
[23] "Caffeine and other adulterants in seizures of street cocaine in Brazil," International Journal of Drug Policy, vol. 14, pp. 331—334, 2003.
[24] "Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes," Journal of Natural Products, vol. 79, pp. 324—331, 2016.
[25] "Pulmonary effects of inhaled cannabis smoke," The American Journal of Drug and Alcohol Abuse, pp. 1—14, 2019.
[26] "Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review," Archives of Internal Medicine, vol. 167, pp. 221—228, 2007.
[27] "Cannabis use and risk of lung cancer: a case—control study," European Respiratory Journal, vol. 31, pp. 280—286, 2008.
[28] "Lower-risk cannabis use guidelines: a comprehensive update of evidence and recommendations," American Journal of Public Health, vol. 107, pp. e1—e12, 2017.
[29] "Cannabis as a cause of death: A review," Forensic Science International, 2019.
[30] "Assessment of dronabinol and its stereo-isomers," World Health Organization (WHO), 2006.
[31] "Fatal intoxication with synthetic cannabinoid MDMB-CHMICA," Forensic Science International, vol. 261, pp. e5—e10, 2016.
[32] "Bad trip due to anticholinergic effect of cannabis," General Hospital Psychiatry, vol. 35, pp. 682—e5, 2013.
[33] "Cannabis (marijuana): Acute intoxication," UpToDate.com, July 2019.
[34] "Effects of Cannabis and the Cannabinoid," in Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential, pp. 61, 2002.
[35] "Inhibition of salivary secretion by activation of cannabinoid receptors," Experimental Biology and Medicine, vol. 231, pp. 1421—1429, 2006.
[36] "HMDB0002845," The Human Metabolome Database 4.0, 2018.
[37] "Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides," PNAS, vol. 109, pp. 12811—12816, 2012.
[38] "Coenzyme A," Nucleic Acids Residues (Database Issue), vol. 39, pp. D1035-41, 2011.
[39] "Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes," Life Sciences, vol. 80, pp. 1415—1419, 2007.
[40] "Glucuronides of monohydroxylated bile acids: specificity of microsomal glucuronyltransferase for the glucuronidation site, C-3 configuration, and side chain length," Journal of Lipid Research, vol. 27, pp. 89—101, 1986.
[41] "Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review," Drug Metabolism Reviews, vol. 46, pp. 86—95, 2014.
[42] "11-Hydroxy-Δ9-tetrahydrocannabinol: pharmacology, disposition, and metabolism of a major metabolite of marihuana in man," Science, vol. 177, pp. 62—64, 1972.
[43] "Intravenous injection in man of Δ9-tetrahydrocannabinol and 11-OH-Δ9-tetrahydrocannabinol," Science, vol. 177, pp. 633—635, 1972.
[44] "Comparative pharmacology of Δ9-tetrahydrocannabinol and its metabolite, 11-OH-Δ9-tetrahydrocannabinol," The Journal of Clinical Investigation, vol. 52, pp. 2411—2417, 1973.
[45] "Interpretation of workplace tests for cannabinoids," Journal of Medical Toxicology, vol. 13, pp. 106—110, 2017.
[46] "Rapid quantification of free and glucuronidated THCCOOH in urine using coated well plates and LC—MS/MS analysis," Bioanalysis, vol. 9, pp. 485—496, 2017.
[47] "Mandatory Guidelines for Federal Workplace Drug Testing Programs: Notice," Federal Register, vol. 73, pp. 71858—71907, 2008.
[48] "Implications of plasma Δ9-tetrahydrocannabinol, 11-hydroxy-THC, and 11-nor-9-carboxy-THC concentrations in chronic cannabis smokers," Journal of Analytical Toxicology, vol. 33, pp. 469—477, 2009.
[49] "A warning against the negligent use of cannabidiol in professional and amateur athletes," Sports, vol. 7, pp. 251, 2019.
[50] "Labeling of Cannabidiol Products: A Public Health Perspective," Cannabis and Cannabinoid Research, 2020.
[51] "Commercial cannabidiol oil contaminated with the synthetic cannabinoid AB-FUBINACA given to a pediatric patient," Clinical Toxicology, vol. 24, 2019.
[52] "Even High Doses of Oral Cannabidiol Do Not Cause THC-Like Effects in Humans: Comment on Merrick et al. Cannabis and Cannabinoid Research 2016, 1(1)," Cannabis and Cannabinoid Research, vol. 2, pp. 1—4, 2017.
[53] "Resorcinol derivatives: a novel template for the development of cannabinoid CB1/CB2 and CB2-selective agonists," Journal of Pharmacology and Experimental Therapeutics, vol. 301, pp. 679—689, 2002.
[54] Figure 2 from, "Cannabidiol: an overview of some chemical and pharmacological aspects. Part I: chemical aspects," Chemistry and Physics of Lipids, vol. 121, pp. 35—43, 2002.
[55] "Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC," Clinical Chemistry, vol. 55, pp. 2180—2189, 2009.
[56] "Decarboxylation study of acidic cannabinoids: a novel approach using ultra-high-performance supercritical fluid chromatography/photodiode array-mass spectrometry," Cannabis and Cannabinoid Research, vol. 1, pp. 262—271, 2016.
[57] "Isolation of Δ9-THCA-A from hemp and analytical aspects concerning the determination of Δ9-THC in cannabis products," Forensic Science International, vol. 149, pp. 3—10, 2005.
[58] "Conversion of CBD to Δ8-THC and Δ9-THC," U.S. Patent 7,399,872, July, 2008.
[59] "Novel methods and related tools for CBD conversion to THC," U.S. Patent App. 16/741,741, July, 2020.
[60] "Decarboxylation of Tetrahydrocannabinolic acid (THCA) to active THC," European Industrial Hemp Association (EIHA), pp. 1-3, 2016.